
A Comprehensive Overview
Saffron (Crocus sativus) is a triploid monocotyledon that produces the world’s most expensive spice. Its main components crocin, picrocrocin and safranal are responsible for its colour, taste and aroma. The saffron plant produces a red-coloured spice that is important in the pharmaceutical, cosmetic, perfume and textile dye industries.
Iran produces nearly 90% of the world’s total production. The saffron market is projected to grow by 12.09% during the forecast period 2020-2027.
This paper reviews current knowledge on the taxonomy, geographical distribution, reproductive biology, chemical composition, therapeutic and traditional uses, and agricultural techniques of saffron, the world’s most expensive spice plant.
1- First of all
Saffron (Crocus sativus L.) is he one of the most expensive spices and is mainly grown as a perennial. The crimson stigma of saffron is used as a spice. Saffron is grown and used in Iran, Spain, India, Italy, Afghanistan, Azerbaijan, United Arab Emirates, Turkey, France, Egypt, Switzerland, Israel, Greece, China, Japan, Iraq and most recently Australia (Tasmania). It has been. Dyes, spices, medicinal.
Global production of dried saffron is estimated at about 418 megagrams (Mg) per year (Cardone et al. 2020).
Iran produces more than 90% of the world’s saffron production, of which over 92% is produced in Khorasan province. In India, saffron is mainly grown in Jammu and Kashmir. Recently, a few cases of saffron cultivation have been recorded in Himachal Pradesh and Uttarakhand.
Saffron is propagated manually by daughter bulbs that are nutritionally produced from the mother bulb.
There are three main factors that give saffron its colour, taste and aroma:
Crocin has strong colouring power, picrocrocin has a bitter taste, and safranal has a unique odour and aroma (Cagliani et al. 2015).
Due to its high price, saffron is widely laced with additives such as corn husks and safflower leaves, making it difficult to trade (Babaei et al. 2014). Iranian saffron is mainly imported from India and Spain.
India imports saffron from major producers. H. Iran, Spain, China. In 2018, India imported US$18.3 million worth of saffron in US currency, making it the world’s fourth largest importer (Anonymous 2019).
Over the past 30–40 years, saffron production has declined, except in Iran, where production has increased
2- Saffron Biology
2.1 Taxonomy
Saffron is a monocotyledonous plant (Iridaceae) native to Southern Europe and Southwest Asia. It is widely used because it is highly adaptable and can be cultivated for a long period of time (Leone et al. 2018). Saffron is taxonomically classified as:
Domain – Eukaryote
Kingdom—Plant
Sub-Kingdom—Trachea
Super Division – Sperm Cell
Department – Magnoliophyta
Class — Lyriopsida
Subclass — Liliaceae
Order – Rereales
Family – Iridaceae
Genus – Crocus
Species – sativus (USDA 2020)
The Crocus genus includes about 80 species worldwide. Among these species, saffron (Crocus sativus L.) is cultivated for its stigma and used as a high-value spice. About eight taxa are currently recognized in Iran (Sharafzadeh 2011).
3 – Habitat
Saffron grows from sea level to almost 2000 m but is better suited to hills, mountain valleys and plateaus at altitudes between 600 and 1700 m. The advantage of this crop is that it can be grown in areas where summer droughts are frequent (Salwee and Nehvi 2013).
4 – Distribution
Saffron is native to Greece, Asia Minor and Persia and is now produced in many countries including Iran, Algeria, Italy, India, France, Russia, Morocco, Persia, Turkey and Spain. Its cultivation spans the globe from 0°E to 90°E (Spain-Kashmir) and 30°-45°N latitude (Persia-England) (Khan et al. 2011).
In India, saffron is grown in the districts of Pulwama, Balamulla, Badgam, Anantnag and Kishtwar in the Union Territory of Jammu and Kashmir (Dhar and Mir 1997). Table 1 shows the major saffron-producing countries in the world.
5- Morphology
The crocus plant bears violet-coloured flowers, the stigmas of which are used as a spice (Fig. 1). The stigma of saffron is dark red to reddish-brown in colour. The style is yellowish brown to yellowish orange. Its odour is strong, characteristic, and aromatic. Its taste is characteristic and bitter.
The stigmas are 25 mm in length and trifid-shaped. The styles are about 10 mm long and are cylindrical in shape (Srivastava et al. 2010). The flowers are hysteranthous and flowering takes place in the month of October. Mother corms are replaced by daughter corms after flowering (Dhar and Mir 1997).
Fig. 1
6 – Reproductive biology
Saffron is a triploid species (2n = 24, x = 8 chromosomes). There is no sexual reproduction and plants divide only by vegetative propagation by tubers. This occurs because chromosome pairing becomes uneven during triploid meiosis and gametes cannot develop (Caiola 2004). Therefore, it does not produce viable seeds (Gresta et al. 2008).
In saffron, vegetative propagation continues until the soil space is filled with daughter bulbs, leading to a gradual decline in flower production (Alonso et al. 2012). Traditionally, only 4-5 daughter tubers are produced per year. Therefore, low reproductive rates and fungal invasion of tubers are obstacles to ensuring sufficient quality planting material (Mushtaq et al. 2014).
7 – Chemical composition
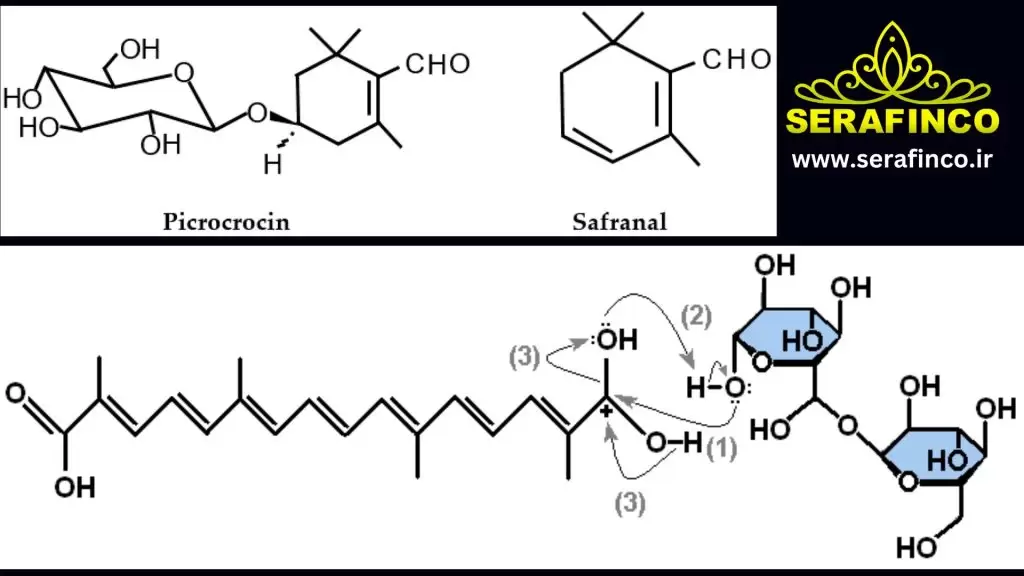
The specific aroma, taste, and colour of a product are due to the presence of secondary metabolites, which are derived from primary metabolites (Parizad et al. 2019). Crocin, safranal, and picrocrocin (Fig. 2) are the main bioactive ingredients of saffron responsible for the colour, aroma, and bitterness of the spice, respectively (Zhang et al. 2019). The chemical characteristics of saffron depend on the different climatic conditions and processing techniques used by the growers.
The chemistry of saffron is complex; this spice has primary metabolites, which are ubiquitous in nature, such as carbohydrates, minerals, fats, vitamins, amino acids, and proteins.
A large number of secondary metabolites, which are products of metabolism that are not critical for survival but important for the development or reproduction of the organism, are also present in saffron, such as carotenoids, monoterpenes, and flavonoids, including mainly anthocyanins (Maggi et al. 2020).
Over 150 constituents that contain hydrophilic and lipophilic carbohydrates, proteins, minerals, mucilage, starch, gums, vitamins, many pigments such as crocin, α and β carotenes, alkaloids, xanthone carotenoid, mangicrocin, saponins, safranal, and picrocrocin have been reported in the stigma of saffron by chemical evaluation (Samarghandian and Borji 2014).
7.1 Crocetin
Crocetin is an unusual lipophilic carotenoid composed of polyunsaturated olefinic acid conjugates. Crocetin’s molecular formula is C20H24O4. The melting point is 285 °C (Samarghandian and Borji 2014). Crocetin has also been documented with the following health-promoting effects: 2016).
7.2 Crocin
Crocin is an important saffron pigment that makes up approximately 80% of the total chemical composition. It is a crocetin diester and is water soluble. Crocin is responsible for the iridescent golden-yellow-red nuances of the spice (Shahi et al. 2016).
The molecular formula of crocin is C44H64O24. Among the carotenoids, water-soluble crocin accounts for 6–16% of the total dry matter content of saffron, depending on the variety selected, environmental conditions, and processing techniques (Gregory et al. 2005).
Crocin 1, also known as α-crocin, a digentioside, is the most abundant crocin in highly soluble saffron (Melnyk et al. 2010). Crocin, which is usually dark red in colour, dissolves quickly in water to a reddish-orange colour, making it useful as a natural food colouring.
Crocin is also known for its antioxidant activity by scavenging free radicals and protecting cells and tissues from oxidation (Papandreou et al. 2006). Unlike unstable safranal, crocin is stable under extreme conditions (Shahi et al. 2016). The λmax of crocin is 440 nm (Kabiri et al. 2017).
7.3 Picrocrocin
The characteristic odour of saffron is mainly derived from picrocrocin, which is present in slightly less weight than crocin. It accounts for about 1–13% of saffron dry matter (Alonso et al. 2001). The molecular formula of picrocrocin is C16H26O7.
When naturally deglycosylated, picrocrocin produces safranal, another important chemical component that is primarily responsible for the saffron odour.
Picrocrocin is a precursor of safranal and monoterpene glycosides. It is responsible for the astringent taste of saffron (Pitsikas 2016; Shahi et al. 2016). The λmax of picrocrocin is 254 nm (Kabiri et al. 2017)。
7.4 Safranal
Safranal is the main constituent of the essential oil of saffron. It is responsible for the aroma of saffron. The molecular formula of safranal is C10H14O. Saffron is a monoterpene aldehyde and aglycon of picrocrocin. It is very interesting to note that fresh saffron stigmas do not contain safranal. It is formed by the action of β-glucosidase on picrocrocin during dehydration and storage after harvest (Maggi et al. 2010; Shahi et al. 2016).
Safranal may make up 70% of the total volatile fraction in some samples. The λmax for safranal is at 330 nm (Kabiri et al. 2017). Spectrophotometry and thermal desorption-gas chromatography are used to measure the value and content of safranal, respectively. Years ago, the safranal content was considered as an index to determine saffron quality; however, this process is no longer acceptable (Aytekin and Acikgoz 2008).
Safranal comprises roughly 30 to 70% of saffron’s essential oil and 0.001 to 0.006% of its dry matter (Carmona et al. 2006; Maggi et al. 2010).
Apart from its traditional use as a spice due to its aroma and flavour, saffron is known to have a high antioxidant potential (Assimopoulou et al. 2005) as well as cytotoxicity towards cancer cells in vitro (Escribano et al. 1996).
8.1 Therapeutic properties
Crocin, the most abundant active ingredient in saffron, has been reported to show promising therapeutic effects on gene expression and apoptosis in cancer cells. Mirajadi et al. (2016) reported that crocetin showed inhibitory effects on cancer cell development because it can reduce the formation of proteins, DNA, and RNA in neoplastic cells.
They also reported an antitumor effect of safranal with low toxicity to normal cells. Today, many people are switching to herbal medicines to avoid the harmful effects of chemicals. Piccus etc. (2008) reported the therapeutic efficacy of crocin in treating anxiety in animals. Tumour development and cancer cell expansion in various testing systems. In vivo and in vitro validated with the saffron extract.
Aung et al. (2007) reported the restriction of colon cancer cell growth by crocin (the main component of saffron) contained in the saffron extract. The anti-tumour properties of the saffron extract are associated with its effects on DNA and RNA formation and its free radical scavenging activity.
In vitro, studies have shown that stigma extract inhibits human tumour cell growth (Shahi et al. 2016). Akhondzadeh et al. (2010) reported the efficacy of saffron extract in treating Alzheimer’s disease. Therefore, active compounds in the saffron extract may help enhance learning and memory (Table 2).
8.3 Common uses of saffron
Saffron is believed to have been first used as a spice and food colouring 3000 to 4000 years ago (Mousavi and Bathaie 2011).
Rather than extracting saffron carotenoids from raw materials and adding them to food, some dishes, such as Indian Prab rice, are cooked with whole or powdered stigma, and water-soluble crocin gives the dish a deep red colour.
Saffron and its preparations have been used as pigments since ancient times. The earliest known example is the use of pigments from dried stigma in cave paintings depicting animals (Humphries 1996) (Table 4).
9 – Agricultural technology
9.1 Geographic distribution
Saffron has been cultivated at different elevations in different geographical locations around the world (Kumar et al. 2009). Crocus genus Distributed from 10° west longitude to 80° east longitude and 30° to 50° north latitude (Yildirim et al. 2017). According to Vavilov, saffron originated in the Middle East (Jan et al. 2014), but some believe that saffron originated in Mediterranean countries.
The ideal altitude for saffron cultivation is 200-2000 m above sea level. More suitable for hills, plateaus and mountains with altitudes between 600 and 1700 m above sea level. In Italy, it is grown at altitudes between 650 and 1100 m above sea level.
In Morocco, it is grown at altitudes between 1200 and 1400 m above sea level (Salwee and Nehvi 2013). Crocus species in India. It thrives in sunny, warm climates and grows best at an altitude of 2140 m above sea level (Menia et al. 2018).
The crop can be grown in temperate, semi-arid and arid regions ranging from 1500 to 2800 m above sea level. Mild winters, warm summers and wet autumns are favourable climatic conditions for high saffron yields (Rahimi et al. 2017).
9.2 Climate requirements
Saffron plants prefer a warm, dry climate with sunny days. Flowering occurs most frequently in October and November, with average temperatures of 15-20°C during the day and 6-8°C at night.
Early autumn rains promote flower production, while spring rains promote bulb proliferation (Menia et al. 2018). It requires warm summers with little or no rain and cool to cold winters with spring rains.
It is cold tolerant (-10 °C), withstanding rare winter snowfalls (Dar et al. 2017). Molina et al. (2010) reported that the effect of temperature is small, with an increase in temperature during flowering slightly reducing the number of shoots per tuber.
The temperature at the time of flowering has a great effect on the size of the flowers. The optimum temperature required for flowering (around 17°C or slightly higher but below 20°C) is much lower than the optimum temperature for germination.
(2010) found that maximum vegetative growth was achieved at a temperature of 27°C and optimal flowering occurred at a temperature of 17°C. Yasmin and Nehvi (2018) reported that an average temperature of 27.5 °C and a total precipitation of 418.90 mm ha-1 were favourable for plant shoot and root development.
Flowering is encouraged in temperate conditions in Kashmir (India) when the average maximum temperature is below 20 °C. At the vegetative stage, plants require 1100 hours of cooling, which is essential for vernalization.
In Kashmir (India), crops receive a maximum average temperature of 11.4 °C, a minimum average temperature of -0.33 °C and 474 mm of precipitation at the vegetative stage.
During the early stages of growth, a temperature range of 23 °C to 25 °C is suitable for vegetative growth, while temperatures below 16 °C are suitable for producing more daughter tubers (Zahmati et al. 2018). In Greece, saffron bulbs grow in March and his April and bloom in September.
and water stress should be avoided during these time intervals (Golmohammadi 2014).
In India, the dry temperate regions of Himachal Pradesh are ideal for cultivation, with temperatures of 12-18°C during the day and 4-5°C at night in September and October. According to Kumar et al. (2009), Palanpur in Himachal Pradesh is an ideal location for saffron cultivation as the average temperature in September and October is 19-23 °C and 8-13 °C.
9.3 Floor requirements
Saffron prefers loose, moist, loose, well-drained clay-limestone soils that are rich in organic matter. 20-30 tons/ha of fertilizer (FYM) is required to increase the organic matter content of the soil (Golmohammadi 2014).
Menia et al. (2018) reported that saffron grows in a wide variety of soil types, but it grows well in well-drained, loose, chalky loamy soils, has a fluffy consistency and roots easily. The best soils for saffron production are sandy or loamy soils. Dal et al.
(2017) observed that saffron thrives in saline soils, but that calcium carbonate deficiency may be a limiting factor. , and the conductivity ranged from 0.09 to 0.30 dS m-1, with an average value of 0.17 dS m-1, suggesting that it is suitable for saffron growth.
9.4 Land preparation
Growing saffron requires proper soil preparation. Plow 3-5 times in May, June and July to a depth of 30 cm to create a crumbly loose texture. Approximately 10 Mg ha−1 of degraded FYM is sufficient for tuber growth (Menia et al. 2018). Maximum amounts of FYM (10 Mg ha−1), P2O5 (60 kg ha−1), K2O (60 kg ha−1) and ¼ of nitrogen (22.5 kg ha−1) should be added.
– Change clothes every 12-15 days. Slurry and fertilizer application should be managed through cross-cultural activities such as tillage, hoeing and land clearing in August of the following year.
9.5 Planting time
Saffron bulbs should be planted from the second half of August to the first half of September. Tubers are sown by hand behind the plow after the bed has formed.
Saffron likes cool winters, wet autumns, spring rainfall, and warm, dry summers. Tuber reproduction is promoted by spring rains, and early autumn rains increase flower production (Menia et al. 2018). According to Bayat et al. (2016), June-July is the best time to sow saffron bulbs in the Mashhad region of Iran.
(2016) reported a gradual decline in saffron growth and flowering index when the planting date was postponed. The highest and lowest flower yields were recorded when the bulbs were planted in June and his October, respectively.
Sowing in spring increases saffron growth and production because the bulbs are dormant. In India, the best time to sow tubers is from the last week of August to mid-September (Husaini et al. 2010). According to Gresta et al. (2016), The best time for sowing saffron tubers in Italy in August. Kafi et al. (2018) reported that the best time to sow tubers is from the last week of August to mid-September.
9.6 Planting spacing/density
Plant density influences stigma yield and biochemical composition of saffron. Dense planting increases the number of plants and buds, increasing the overall yield. High bulb density results in large stigmas and large buds.
The total yield is influenced more by flower number than by stigma weight (Andabjadid et al. 2015). (2014) reported that dense planting of tubers increased flower and tuber yields. When planting 300 (1st year) or 200 (2nd year) tubers m-2, 4-6 g mother tubers produced the maximum number of flowers.
Still, depending on the size of the mother tuber, the planting density may be below or above his 200 tubers m-2. According to Mohammad et al. (2011), a planting pattern of 10–20 cm increased the fresh dry stigma yield (12 kg ha-1), flower yield (170 kg ha-1), average tuber weight (9.2 g), and average tuber diameter ( 1.5 cm). In Spain, tubers are planted in trenches 20 cm deep, at intervals of 8-10 cm or 12-15 cm.
It is planted in two rows. Tubers are covered with soil from adjacent furrows at 30–35 cm intervals (Kafi et al. 2018). In Kashmir, rectangular strips 1.5–2 m wide and 2–3 m long are used with drainage lines 30 cm wide on each side and 20 cm deep (Husaini et al. 2010).
These raised beds are planted with tubers 12–15 cm deep with 10 × 20 cm spacing between tubers and furrows (Munshi et al. 2001).
9.7 Comb course
The amount of tubers needed to plant one hectare depends on tuber size, growing period and distance. Tubers weigh about 2.5–3.0 Mg, or about 500,000 tubers with an average diameter of 2.5 cm are required per hectare (Kumar et al. 2009).
According to Kafi et al. (2018) In conventional systems, tubers are sown in mounds at 25-cm intervals and may be randomly planted with up to 15 tubers per mound. Flatbed seeding has also been reported to be more advantageous than furrow seeding (Kafi and Showket 2007).
9.8 Planting spacing/density
Plant density influences stigma yield and biochemical composition of saffron. Dense planting increases the number of plants and buds, increasing the overall yield.
High bulb density results in large stigmas and large buds. The total yield is influenced more by flower number than by stigma weight (Andabjadid et al. 2015). (2014) reported that dense planting of tubers increased flower and tuber yields.
When planting 300 (1st year) or 200 (2nd year) tubers m-2, 4-6 g mother tubers produced the maximum number of flowers. Still, depending on the size of the mother tuber, the planting density may be below or above his 200 tubers m-2.
According to Mohammad et al. (2011), a planting pattern of 10–20 cm increased the fresh dry stigma yield (12 kg ha-1), flower yield (170 kg ha-1), average tuber weight (9.2 g), and average tuber diameter ( 1.5 cm). In Spain, tubers are planted in trenches 20 cm deep, at intervals of 8-10 cm or 12-15 cm. He is planted in two rows.
Tubers are covered with soil from adjacent furrows at 30–35 cm intervals (Kafi et al. 2018). In Kashmir, rectangular strips 1.5–2 m wide and 2–3 m long are used with drainage lines 30 cm wide on each side and 20 cm deep (Husaini et al. 2010).
These raised beds are planted with tubers 12–15 cm deep with 10 × 20 cm spacing between tubers and furrows (Munshi et al. 2001).
9.7 Comb course
The amount of tubers needed to plant one hectare depends on tuber size, growing period and distance. Tubers weigh about 2.5–3.0 Mg, or about 500,000 tubers with an average diameter of 2.5 cm are required per hectare (Kumar et al. 2009).
According to Kafi et al. (2018) In conventional systems, tubers are sown in mounds at 25-cm intervals and may be randomly planted with up to 15 tubers per mound. Flatbed seeding has also been reported to be more advantageous than furrow seeding (Kafi and Showket 2007).
9.9 Nutritional management
Saffron does not require high levels of nutrients. The use of high amounts of fertilizer, especially nitrogen fertilizer, promotes growth but reduces yield (Kafi et al. 2018).
Saffron yield is highly sensitive to soil fertility levels (Mohammad et al. 2012). The use of 20-30 t/ha of organic fertilizer is the most common fertilization method worldwide (Kocheki 2003).
Sameer et al. (2018) reported that the use of Trichoderma rides along with vermicompost on soil already treated with neem cake in semi-annual planting cycles could control cork rot, according to Mohamad et al. (2012), He applied 20–30 Mg ha-1 FYM in combination with chemical nitrogen (23 kg ha-1) significantly improves soil fertility.
A mixture of cow dung (20 Mg ha-1) and urea (50 kg ha-1) yielded the highest yield (0.45 g m-2), highest fresh flower weight (0.89 g) and longest stigma (29 mm). Applying nitrogen increases vegetative growth but does not significantly increase yield.
In traditional saffron cultivation, large amounts of FYM are added during cultivation, totalling about 20-30 Mg ha−1. FYM provides nutrients, increases soil water holding capacity and improves soil structure in non-irrigated conditions.
After planting the tubers, the field is not fertilized at all (Dar et al. 2017). Iranian farmers use FYM (10–80 Mg ha−1), but after the first irrigation in early autumn, soil crusts were first decomposed at concentrations of 100 kg ammonium phosphate and 100 kg urea ha−1.
Inorganic fertilizers are also used after 1 weeding (Behnia 2008; Ghorbani and Koocheki 2017). (2009) observed that inorganic and organic fertilizers increased quantitative and qualitative saffron yields. Alidadi et al.
(2013) reported a 10.2% increase in saffron yield when vermicompost was increased from 4 Mg ha–1 to 8 Mg ha–1. Increasing the granular sulfur compost from 4 Mg ha–1 to 8 Mg ha–1 decreased saffron yield by 70.8% due to increased electrical conductivity. Therefore, the main reason for the decrease in saffron yield can be attributed to its high electrical conductivity.
Jamie Al. (2020) reported that using earthworm compost at 24 Mg ha−1 and mycorrhizae at 400 kg ha−1 increased the number of flowers by 28.08% in the second year.
9.10 Water management
Saffron is suitable for arid and semi-arid regions as it undergoes a 5-month dormancy period during which the tubers do not require water, starting in early May when the spring rainfall is almost over (Kafi et al. 2018). Mosaferi (2001) reported that mid-June watering reduced saffron yield by 17%, while summer watering in late August increased flower yield by about 20%. I’m here.
The risk of fungal diseases is usually increased by summer watering. Additional basin irrigation should be used for watering. In autumn, it tends to get less rain, so you need to water about 100mm before flowering.
In areas with 600mm of seasonal rainfall, about 50mm of post-flowering irrigation provides sufficient economic performance. Areas with seasonal rainfall of 400 or 200 mm require additional irrigation at intervals of 24 or 15 days or 50% ETp (potential evapotranspiration) and 75% ETp irrigation systems (Sepaskhah and Kamgar 2009).
(2014) showed that when applying only 50% of the saffron water requirement (BWR) in the first year, the floral properties were unaffected. However, when only 50% of SWR was applied compared to 75% or 100% of SWR, the number of flowers and yield of dry stigmas decreased significantly in the second year.
Sprinkler irrigation of 700 m3 ha−1 increased saffron productivity by 40% (Nehvi and Makhdoomi 2007).
9.11 Weed management
Weed control is essential for healthy crop growth. Annual crops in Italy are hand weeded, while perennial crops are managed with the herbicides simazine (Gesatop 50) or atrazine (Gesaprim 50) at 1.0 kg ha−1 (Dar et al. 2017). It is difficult to use twist and finger weeders on saffron, mainly due to the presence of new tubers outside the first harvest row.
Rocky soils are also an obstacle for these instruments (Cirujeda et al. 2014). Zareh Hosseini and others in a saffron field in Gonabad, Iran. (2014) reported that the only dominant weed species were grey cress (Cardaria draba), mouse barley (Hordeum murinum), wild golden barley (Hordeum spontaneum), and yarrow (Achillea millefolium).
In India, the vegetative growth of saffron takes place from October/November to April, and the dormant season of saffron, from May to September, makes glades prone to weeds.
The main weed species reported in the saffron fields of J&K (Jammu and Kashmir Federal Region), India, are Chenopodium album, Tulipa stellata, Papaver rhoae, Lepidium virginicum, Euphorbia helioscopia, Salvia moorcroftiana, Filago arvense and Garium tricorne. , Polygonum aviculare, and Erodium. cicutarium, Rananculus arvensis, Medicago lupilina, Poa bulbosa, Lithospermum arvense (Husaini et al. 2010).
Ioxynil (750 g actives [grams actives] ha-1) and tibolone-methyl (18.75 g actives ha-1) at the leaf stage after the 6th to 8th spray harvest were very effective for weed control. It was effective. Metribuzine (560 g a.i. ha−1) is widely used in spring or autumn for weed control without harming saffron (Menia et al. 2018).
9.12 Harvest
When the saffron is harvested, the flowers are picked and the stigma is removed. Flower assembly begins when they appear in the field (Kafi et al. 2018).
Flower gathering starts in October to November in Khorasan Iran, but varies from region to region depending on climate change and the initial watering season (Kafi and Showket 2007). Early morning flowers are picked mainly by the women of the family, to a lesser extent by Kashmir’s employed workers.
The picked flowers are carried under the roof to separate the stigmas and flowers. The detached flower parts are dried in the shade (sunny day) for a few more days (Husaini et al. 2010). The flowering period in Italy is from mid-October to November 10th.
In Spain, this day is known as “Mantelpiece Day”. H. is the time of greatest expulsion during flowering, and the landscape seems to be wrapped in a cloak of flowers. Dal et al. (2017) reported that saffron usually blooms in autumn about 40 days after planting, depending on the weather.
9.13 Plant Productivity
Flower size and the proportions of different parts of the flower are influenced by many environmental and genetic factors. In Khorasan, 78.5 kg of fresh flowers (approximately 170,000 flowers) are required to produce 1 kg of dry stigma and style (Husaini et al. 2010).
Dal et al. (2017) reported that tuber size has a significant effect on the number of flowers per tuber. Per bulb, she produces 12 flowers with bulbs over 45 g of her and an average of 6 daughter bulbs with 20-30 g bulbs.
It is recommended to pick the flowers daily at dawn when the crowns are still closed to avoid loss of colour and quality, avoid sudden wind and rain loss, and make it easier to remove the floral components. (Tammaro 1990).
On the saffron farm, he only produces 3 flowers per plant, which must be harvested by hand every day. 1 kg of dried saffron is made from 78 kg of fresh flowers, and about 2170 flowers make 1 kg of fresh flowers. Stigma is the final product of commercial value (Emadi 2009).
10 – Post-harvest management
10.1 Drying
Post-harvest drying of stigmas plays an important role in determining saffron quality. Many researchers have studied dryness and scar quality, with mixed results.
Acare etc. (2011) compared the quality of saffron by evaluating crocin and safranal in freeze-dried stigma (4 h at -40 °C) and sun-dried stigma (18 °C ambient temperature). Their results showed that safranal and crocin were higher in freeze-dried scars. Feili et al.
(2012) used the indirect sun-drying method and compared the quality of dried wounds with conventional methods. They reported that the quality of sun-dried scars was superior to conventionally dried scars. Chaouqi et al.
(2018) showed that drying saffron stigmas in an oven at 40 °C is superior to conventional methods. Che et al. (2020) used different drying methods, including vacuum drying, freeze drying, microwave drying, oven drying, and infrared drying, and found that crocin I was highest in oven-dried samples and lowest in infrared-dried samples. bottom.
Crocin II was highest in freeze-dried samples. From the above experiments, it can be concluded that frozen and oven-dried stigmas are of higher quality than conventionally dried stigmas, but this method is not cost-effective and May not be suitable for limited farmers.
10.2 Storage
Proper storage of saffron bulbs is important to prevent germination (Kumar et al. 2009). Kashmiri saffron bulbs are stored in clay pots or plastic bags regardless of moisture content (Mir et al. 2008).
Saffron with an initial moisture content of 8–10 °C can be safely stored in an airtight container at an ambient temperature of 10 °C for 6 months (Menia et al. 2018). Storing tubers at 30 °C leads to high stigmatization and production of daughter tubers (Siracusa et al., 2010). Cavusoglu (2010) conducted an experiment in which at 8 °C he compared tubers stored for 7–28 days with tubers stored under controlled conditions at room temperature.
He reported that yield attributes (the number of flowers, formation of dry and fresh stigmas) decreased significantly with longer storage times (up to 28 days) for tubers stored at 8 °C. bottom. Hajizadeh et al. (2017) observed that tubers should be stored at 25 °C to achieve high yields.
11 – Contamination
As saffron is an expensive commodity, it is subject to various forms of fraud by traders. Food adulteration involves the addition of any inexpensive ingredient to an expensive or valuable product so that the product is produced at the lowest cost and maximum profit.
The most common type of saffron production scam is the introduction or blending of similar products such as safflower, red silk fibres, turnips, marigolds with red stigmas, and pomegranates (Heidarbeigi et al. 2015). Many researchers have worked on methods to detect various types of contamination in saffron.
Varliklioz Er et al. (2017) laser-induced decomposition spectroscopy (LIBS), attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), and principal component analysis ( developed and tested Raman spectroscopy using PCA.
LIBS technique and PLS showed that plant contamination susceptibility was sensitized with saffron below 10 μn. This is difficult to detect with UV-Vis reference spectroscopy systems. Petrakis and Polissiou (2017) used diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and chemical measurements to identify the six characteristic plant contaminants of turmeric, gardenia, C.
sativus stamens and marigolds. tested for saffron contamination They concluded that DRIFTS is a safe, low-cost alternative using chemometric analysis to rapidly assess saffron contaminants, including plant-derived components. Petraki et al. (2015) assessed saffron contamination with plant products using 1H NMR metabolite fingerprinting by applying both the OPLS-DA and O2PLS-DA models to his 1H NMR data.
They concluded that NMR metabolite fingerprinting is more efficient than the ISO 3632 method for determining saffron, especially powdered contaminants.
12 – Marketing
Marketing channels are important because they support and streamline how each consumer gets the products they want (Qadri 2018). Channel composition is a major research area in marketing today, and existing research has identified unhealthy market channels for saffron (Hamid et al. 2017).
Producers with asymmetric knowledge of market conditions and poor transportation and storage infrastructure suffer severe losses (Ganie and Nusrath 2016). Kheirandish and Gowda (2012) found that the number of arbitrators decreased, and governments offered a proactive approach to developing marketing and reported that they have great potential as they use such syndicates as high channels.
Increase the share of producers in consumer prices. In Kashmir, India, farmers sell their produce through middlemen and suffer heavy losses due to a lack of knowledge of end-market supply and demand and financial instability (Ali and Hakim 2017).
Hamid et al. (2017) concluded that farmers incurred marketing costs of 2,41,744 kg-1 and physical losses of 44,876 kg-1. Marketing costs for wholesalers were 2,54,960 ₹ kg-1 and for retailers were 2,60,223 ₹ kg-1. The wholesaler’s physical loss was 26,717 ₹ kg-1, while the retailers was 15,027 ₹ kg-1. The wholesaler’s marketing margin was 13,823 kg−1 and the retailer’s margin was 1,46,431 kg−1. Marketing channels play an important role in determining farmers’ profits and losses. Therefore, farmers should exercise extreme caution and acquire proper knowledge of market practices.
Process
13 – Saffron Petals
As only the stigma of the saffron flower is used economically, the rest of the flower is considered waste or used as fertilizer (Zeka et al. 2015). However, researchers report that other parts of the flower have economically exploitable and beneficial activities for growers.
Zeka et al. (2015) reported the presence of kaempferol (126 mg g-1 dry weight, much higher than in broccoli) which exhibits antioxidant activity and can be used as a dietary supplement. In fact, the antioxidant activity and presence of kaempferol have been reported by many researchers (Menghini et al. 2018; Montoro et al. 2012; Rigi et al. 2015; Serrano Diaz et al. 2014, 2012) in different ways.
Khoshsang and Ghaffarinejad (2018) reported the efficacy of saffron petals as a solvent to remove harmful her Pb2+ ions from wastewater.
From the above studies, it can be concluded that saffron flowers, which are now considered a waste product, are very likely to be used in various ways in the pharmaceutical and food industries. The development of these uses will be a boon to saffron farmers.
14 – Conclusion
Saffron is an expensive spice plant grown mainly in Iran. Used in the food and colouring industry worldwide. Many researchers have also demonstrated its therapeutic properties, so it can be used as a medicinal plant, although it may not be very cost-effective.
The demand for this spice plant in India is increasing and its Portions are met by imports from countries like Iran, putting a heavy strain on the national coffers.
To meet demand and reduce imports, India needs to increase the acreage of this crop. In this regard, the CSIR Himalayan Bioresources Technology Research Institute in Palanpur, Himachal Pradesh introduced saffron as a crop in non-traditional areas across India in 2018-19 and 2019-20 (Figure 4).
Since only the stigma of the saffron flower is used, the rest of the flower is lost. Not much work has been done to use the remaining flower parts, so this will be an important aspect of further research.
